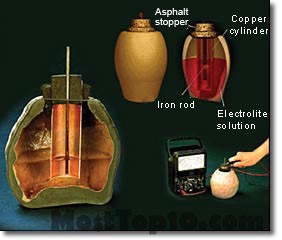
In 1951, German archaeologists found tools from the Parthian period (250 BCE to 224 CE) near Ctesiphon. After examination, it was found that these tools are electric cells that were made and used by Iranians.
He published this found in an article, describing the device as an ancient battery used for plating and transfer a layer of silver and gold from surface to surface.
Examples of Ctesiphon batteries
In 1801, the French physicist N. Gautherot connected the two electrodes of a Volta cell to two platinum wires immersed in saline solution and passed electric current through them [1]. Water decomposed to hydrogen and oxygen, and when the circuit was cut off and the platinum wires were connected to each other, electric current flowed in the opposite direction for a short time.
A year later in Germany, Johann Ritter connected a Volta cell to layered discs of copper and cardboard moistened with NaCl solution [2]. The charging voltage was 1.3 V. After the circuit was disconnected, a voltage of 0.3 V was measured between the two copper discs. Ritter conducted similar experiments with lead, tin and zinc plates. Different voltages were measured for the different types of plates. He called this voltage polarization.
When electric current flows through lead electrodes immersed in H2SO4 solution, lead dioxide is formed on one of the electrodes. Such experiments were performed by Ka ¨stner in 1810, Nobili in 1828, Scho ¨nbein in 1838 and Wheatstone in 1843.
In 1854, the German medical officer Wilhelm Sinsteden, while studying the electrical phenomena, observed that when electric current flowed through lead electrodes immersed in diluted H2SO4 solution the obtained cell delivered electric current and had a specific energy output of 0.1 Wh kg1 for 15 min of discharge.
Gaston Plante ´ e The Inventor of the Lead Acid Battery
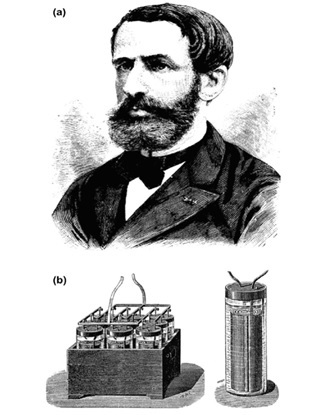
In 1859, the French physicist Gaston Plante ´ studied the polarization between two identical electrodes immersed in diluted aqueous solution of sulfuric acid. He investigated different electrodes including silver, lead, tin, copper, gold, platinum and aluminum electrodes. He established that, depending on the type of electrodes used, the cells were polarized to different levels when electric current flowed through the electrodes and the cells became generators of reverse current. He summarized the results of all these experiments in his paper entitled ‘Recherche’s sur la polarization voltaique’, which was published in Competes Rendus of the French Academy of Sciences in 1859 . Gaston Plante ´ established that the secondary current (as was then called) that flowed through a cell with lead plates separated by rubber strips and immersed in 10% sulfuric acid solution was the highest and flowed for the longest period of time as compared to all other cells under test. This cell had the highest voltage, too. On 26 March 1860, Gaston Plante ´ demonstrated before the French Academy of Sciences the first rechargeable lead acid battery comprising nine cells connected in parallel and presented a lecture entitled ‘Nouvelle pile secondaire d’une grande puissance. This was practically the birth certificate of the lead acid battery.
In 1989, on the eve of the 130th anniversary of the invention of the leadeacid battery and in connection with the first LABAT international conference on leadeacid batteries, the Bulgarian Academy of Sciences, with the approval of the French Academy of Sciences, established an award, a medal, named after the great French scientist Gaston Plante ´.
The Gaston Plante ´ Medal is presented every 3 years to prominent scientists who have made significant contributions to the development of lead acid battery science and technology. The recipients of the award are elected by an international committee comprising 15 scientists from all over the world. Up to now the Gaston Plante ´ Medal has been awarded to 11 scientists from 7 countries as well as to the Advanced Lead Acid Battery Consortium for promoting research and development in the field of lead acid batteries.
What Pains Had the Lead Acid Battery to Go Through?
1-The lead acid battery was born some 10 years before the invention of the mechanical generators of electricity. Being a secondary power source, the lead acid battery needed a cheap and easy-to-use charging device. At that time, the battery was charged by Daniell cells or Bunsen cells, which was not an easy procedure at all.
2- In 1879, Thomas Edison in the United States and Joseph Swan in England invented the incandescent lamp, thus making it possible to convert electric energy into light. The invention of the light bulb promoted the use of electricity in people’s everyday life. Electricity entered human lives. Lead acid battery demand increased. However, largescale production of lead acid batteries was constrained by the technology of their manufacture. Immediately after its invention, the lead acid storage battery attracted the interest of many researchers who looked for variants of Plante ´’s cell.
First Applications of Lead Acid Batteries in Human Life
Thus, in the early 1880s, a lead acid battery of high capacity and relatively simple technology of manufacture was created. This battery rapidly found various practical applications.
In 1881, Gustave Trouve ´ first used a lead acid battery in his three-wheeled electric automobile, which reached a speed of 12 km h1. In 1886, the first submarine propelled by lead acid batteries were launched in France. A lead acid battery was mounted in a small dirigible balloon which was propelled at a speed of 4 m s1. In 1899, Camille Jenatzy reached a speed record of 109 km h1 with his cigar-shaped electric car powered with lead acid batteries.
The lead acid battery was quickly adopted in the emerging telecommunications technologies:
first, in Morse electric telegraph and later by the telephone companies in the United States, too.
In 1882, the city of Paris installed a system for the distribution of electricity for lighting comprising a combination of dynamos and lead acid batteries. The first electrically lit street was Grands Magasins du Louvre in Paris (‘the city of lights’). In 1883, Plante ´ supplied the Imperial Palace of Franz Josef in Vienna with stationary and portable equipment for lighting. The lead acid battery was gradually deployed in various sectors of the industry and became an important means of electric energy production and storage. Thus, the first stage in the development of the lead acid battery system and the technology for its manufacture was completed.
Position of the Lead Acid Battery Among the Secondary Electrochemical
The lead acid battery could be adequately evaluated only if compared to the other types of secondary power sources. A theoretical assessment can be made by comparing the electrical, energetic, power and economic parameters of the different sources of electricity, but the relative share of each battery chemistry in practical applications is the most objective evaluation criterion. Table 1.1 summarizes the basic energy and power characteristics of six types of secondary power sources which are currently used most widely. Data from the studies of Wentzl [78] and Ko ¨hler [79] have been used. It can be seen that the lead acid battery has inferior specific energy and power characteristics as compared to the other types of batteries.